The Heat is on! A huge amount of the technology and comforts that we have in our world just wouldn’t exist if it wasn’t for what we’ve learned about the way heat behaves. So keep cool and use this excellent series to teach your students everything that they need to know about heat, including its effect on things and how it transfers from one thing to another.
In Episode 6, Heat and the Human Body, we look at the brilliant systems within our bodies that allow us to maintain a more-or-less constant body temperature regardless of the weather conditions. Sweating, shivering, pumping more blood to some parts of our bodies than to other parts, curling up into a ball, and spreading out your arms and legs all play their part at keeping our bodies at the right temperature! But what happens when we reach our limits?
A 3-minute excerpt followed by a 2 minute summary.
 The Episode 6 Question Sheet for Students:
The Episode 6 Question Sheet for Students:
![]() The PDF version.
The PDF version. ![]()
Google The Google Doc version. Google
Get the answers.
![]() If you have ClickView, watch the whole episode here.
If you have ClickView, watch the whole episode here.
![]() If you have Learn360, watch the whole episode here.
If you have Learn360, watch the whole episode here.
![]() If you have Films on Demand, watch the whole episode here.
If you have Films on Demand, watch the whole episode here.
![]() If you have Classroom Video, watch the whole episode here.
If you have Classroom Video, watch the whole episode here.
![]() Most of our videos are also available on SAFARI Montage. Just log in and do a quick search.
Most of our videos are also available on SAFARI Montage. Just log in and do a quick search.
![]() Don’t have any of the above? Rent or buy the Shedding Light series and/or individual programs from our Vimeo page!!
Don’t have any of the above? Rent or buy the Shedding Light series and/or individual programs from our Vimeo page!!
The Transcript (which can be used as a textbook)
Contents:
Part A: Introduction. Humans (and all mammals and birds) are endothermic; we can keep our internal temperature the same regardless of the conditions.
Part B: Changing our Surface Area. Why do we spread out our arms and our legs when we’re feeling hot but fold our arms over our chest when we’re feeling cold?
Part C: Blood. Blood doesn’t just carry oxygen and other chemicals around in our bodies. It also carries heat energy around so it can take heat energy from one part of the body to another.
Part D: Sweating and Shivering. Why do we shiver when we’re feeling cold? How does sweating cool us down when we’re feeling hot? How much sweat do you actually lose on a hot day?
Part E: Hypothermia and Hyperthermia. Though our bodies can maintain a constant core temperature, there’s a limit to what we can handle. If our internal temperature rises too much or falls too much we can die. We’ll show you how easily it can happen.
 Part A: Introduction:
Part A: Introduction:
37°C. The human body, regardless of the conditions, how hot or cold it is or how much exercise we’re doing, maintains a temperature of 37°C + or – 0.5°C. But how can it do this even when the sun is beating down on us, or when we’re surrounded by ice-cold air?
 In our last episode we saw that heat energy can transfer from a hotter object to a colder object in three ways: conduction, convection, and radiation.
In our last episode we saw that heat energy can transfer from a hotter object to a colder object in three ways: conduction, convection, and radiation.
When, in Episode 1 of the Shedding Light on Heat series, I placed a container of warmer water into a container of cooler water, heat transferred by conduction from the warmer water into the cooler water. The cooler water’s temperature increased because heat energy was transferring into it, while the warmer water’s temperature decreased, because of course heat was transferring out of it.
In winter, we are typically surrounded by cold air, and heat transfers out of our warm bodies into the cold air.

 Our skin is made of a number of different layers and components but one of the components is a system of temperature-sensitive nerves, coloured yellow here, which spread out in a layer just under the surface. As our skin cools down in the cold air, certain proteins in these nerves vibrate with less and less energy, and this causes a signal to be sent off to the brain telling our brain “it’s cold”.
Our skin is made of a number of different layers and components but one of the components is a system of temperature-sensitive nerves, coloured yellow here, which spread out in a layer just under the surface. As our skin cools down in the cold air, certain proteins in these nerves vibrate with less and less energy, and this causes a signal to be sent off to the brain telling our brain “it’s cold”.
However, despite the fact that it was only 0°C here, and I was ridiculously underdressed for about 10 minutes, my core body temperature was a warm 36.7°C. It didn’t really drop at all.
 And now many months later, the air temperature is 32°C and the sun’s rays are hitting me and my core body temperature is 36.8°C. It’s barely changed at all.
And now many months later, the air temperature is 32°C and the sun’s rays are hitting me and my core body temperature is 36.8°C. It’s barely changed at all.
Our bodies maintain a core temperature of 37 + or – 0.5°C whether it’s hot or whether it’s cold.
Humans (and in fact mammals in general and birds) are endothermic, which is sometimes referred to as being warm blooded. 37 ± 0.5°C
 Being endothermic, we generate heat within our bodies to keep warm and we have mechanisms within our bodies that allow us to maintain a more-or-less constant temperature (37 + or – 0.5°C) regardless of the conditions. Our skin changes temperature, here in the snow, my exposed skin was only 27°C [have footage of thermometer in snow on my arm] but our core temperature, that is the internal temperature of our muscles, our internal organs, our heart, and most importantly our brain stays more-or-less the same and rarely rises above or drops below this range.
Being endothermic, we generate heat within our bodies to keep warm and we have mechanisms within our bodies that allow us to maintain a more-or-less constant temperature (37 + or – 0.5°C) regardless of the conditions. Our skin changes temperature, here in the snow, my exposed skin was only 27°C [have footage of thermometer in snow on my arm] but our core temperature, that is the internal temperature of our muscles, our internal organs, our heart, and most importantly our brain stays more-or-less the same and rarely rises above or drops below this range.
Obviously heaters and air-conditioners and clothing and hot food and cold food and the beach all help us maintain our core body temperature at about 37°C, but our bodies stay at about 37°C even if we’ve got none of these things.
As long as we have enough water to drink, humans can maintain a core temperature of 37°C for hours even if the air temperature is well over 30°C, as it is today.
We can also cope, even if we’re not wearing appropriate clothing, with air temperatures of as low as 10 to 15°C for hours before our core temperature starts to drop.
Maintaining a constant core temperature ensures that all the chemical processes that are going on in our bodies work properly. So, how do our bodies manage to maintain a constant core temperature regardless of the conditions? And what happens when we reach our limits and our core body temperature rises too high or drops too low. Well that’s what we’re going to look at in this episode of the Shedding Light on Heat series. So let’s begin.
 Part B: Changing Our Surface Area
Part B: Changing Our Surface Area
At a simple level, maintaining a constant core temperature requires our bodies to balance the amount of heat energy that we’re producing (within, as we burn fuel) and the amount of heat energy that we’re receiving (from, say, the sun) with how much heat energy that we’re losing to our surroundings. The heat loss has to be equal to the heat gain. If for example we receive too much heat in hot weather, then our bodies have to increase the amount of heat that we lose to our surroundings. Our bodies have ways of increasing heat loss when we’re hot and reducing heat loss when it’s cold.

 When it’s cold, our bodies can lose heat faster than the rate that we’re generating heat. We therefore try to reduce how quickly the heat that we’re generating leaves our bodies. One of the first things we do, assuming we don’t have access to heaters or more appropriate clothing, is to fold our arms over our chest and to bring our shoulders up. We also, if we’re cold at night, scrunch up into a ball. These actions reduce the surface area of our skin that is in contact with the air.
When it’s cold, our bodies can lose heat faster than the rate that we’re generating heat. We therefore try to reduce how quickly the heat that we’re generating leaves our bodies. One of the first things we do, assuming we don’t have access to heaters or more appropriate clothing, is to fold our arms over our chest and to bring our shoulders up. We also, if we’re cold at night, scrunch up into a ball. These actions reduce the surface area of our skin that is in contact with the air.
The only way heat can escape an object is through the surface of the object. If you reduce the surface area, then you reduce how quickly heat can escape, and so the object loses heat more slowly. By covering my chest with my arms, I slow the rate of heat loss and so I stay warmer. Any heat that would have been lost through my chest is trapped and stays inside me. Likewise, any heat that would have been lost through the undersurfaces of my arms is also trapped.

 Let me demonstrate the effect that surface area has on heat loss. Here I have two plastic containers, one wide and flat and the other tall and narrow.
Let me demonstrate the effect that surface area has on heat loss. Here I have two plastic containers, one wide and flat and the other tall and narrow.
In this experiment, I poured exactly 1 kg of hot water (which is 1 litre of water) into the containers and then recorded the temperature of the water every minute for 10 minutes. The water in the wide open container with the larger surface area cooled down a lot quicker than the water in the more squarish container that had less surface area. After 10 minutes, the temperature of the water in the container with the larger surface area fell by 40°C (from 87 to 47), whereas the temperature of the water in the container with the smaller surface area fell by only about 15°C (from 87 to 73). That’s obviously a big difference.
 If I approximate the shape of the water in the two containers, this is the water, not the actual containers, we can calculate the surface area of the exposed top surface of the water in both containers.
If I approximate the shape of the water in the two containers, this is the water, not the actual containers, we can calculate the surface area of the exposed top surface of the water in both containers.
Area (in this case the surface area of the top surface) is simply length times width. The first container’s top surface had a surface area of 8.4 cm × 11.5 cm which is 96 cm2. The second container’s top surface had a surface area of 27.7 cm × 27.7 cm which is 767 cm2, a huge difference. Heat energy can escape into the air more quickly if the surface area is larger.
 The overall surface area (of all six sides) of the water in each container can be calculated by adding up the surface areas of each of the six sides, the three we can see and the three that we can’t and we get 603 cm2 in the top container but nearly 1700 cm2 in the bottom container. So, though we had the same mass of water that started at the same temperature, the water that had the larger surface area cooled down much more quickly compared to the other one because it had a larger surface area through which the heat can transfer out of it.
The overall surface area (of all six sides) of the water in each container can be calculated by adding up the surface areas of each of the six sides, the three we can see and the three that we can’t and we get 603 cm2 in the top container but nearly 1700 cm2 in the bottom container. So, though we had the same mass of water that started at the same temperature, the water that had the larger surface area cooled down much more quickly compared to the other one because it had a larger surface area through which the heat can transfer out of it.
When we fold our arms, we reduce the surface area through which the heat energy in our bodies can escape into the air, and so we don’t get as cold.
We even reduce our surface area in our sleep without even being aware of it. Even babies do it. If there are other people around, we reduce our surface area even more effectively by huddling together.

 In hot weather on the other hand, we don’t lose heat as quickly, so in an attempt to get rid of excess body heat, we increase our surface area by spreading our arms and legs apart. This allows more surface area of our skin to make contact with the air, and so we lose heat at a greater rate. We do it in our sleep, once again without even being aware of it, and even babies do it and it’s not as if they know anything about Physics!
In hot weather on the other hand, we don’t lose heat as quickly, so in an attempt to get rid of excess body heat, we increase our surface area by spreading our arms and legs apart. This allows more surface area of our skin to make contact with the air, and so we lose heat at a greater rate. We do it in our sleep, once again without even being aware of it, and even babies do it and it’s not as if they know anything about Physics!
 Now there are many factors that affect how quickly heat energy transfers from one object to another (that is the rate of heat transfer). The one we’ve just looked at is surface area. There are three others important factors.
Now there are many factors that affect how quickly heat energy transfers from one object to another (that is the rate of heat transfer). The one we’ve just looked at is surface area. There are three others important factors.
The thickness of the material is one of them. In winter, we wear thicker clothes which slow down the rate that heat energy escapes into the air and in summer we wear lighter (that is, thinner) clothing that allows heat to escape faster.
The type of material is also a huge factor. We saw in our last episode that some things conduct heat better than others. Clothing is made of good insulators like cotton and wool. Dolphins, seals, and other marine mammals have thick layers of fat under their skin which trap heat inside their core. The layers of fat are kind of like clothing.
The fourth factor is the temperature difference between the two objects.
The fact that the temperature difference between two objects affects the rate of heat transfer becomes clear if we re-examine the results of our surface area experiment.
 When the water was hot, its temperature decreased rapidly, which means that it was losing heat energy quickly. As it cooled down and got closer and closer to the air temperature in the room its temperature decreased slowly which means that it was losing heat energy slowly. It was still cooling down, but not as quickly. So what does this mean? It means that the rate of heat transfer depends in part on the temperature difference between the two objects. When the temperature difference is high, heat energy transfers quickly, but when the temperature difference is low, heat energy transfers slowly.
When the water was hot, its temperature decreased rapidly, which means that it was losing heat energy quickly. As it cooled down and got closer and closer to the air temperature in the room its temperature decreased slowly which means that it was losing heat energy slowly. It was still cooling down, but not as quickly. So what does this mean? It means that the rate of heat transfer depends in part on the temperature difference between the two objects. When the temperature difference is high, heat energy transfers quickly, but when the temperature difference is low, heat energy transfers slowly.
 So, for example when it’s only 2°C, like it is now, there’s a fairly large temperature difference between the temperature of our bodies and the temperature of our surroundings. We therefore lose heat fairly quickly and our bodies feel cold.
So, for example when it’s only 2°C, like it is now, there’s a fairly large temperature difference between the temperature of our bodies and the temperature of our surroundings. We therefore lose heat fairly quickly and our bodies feel cold.
On a 30°C day, we lose heat very slowly and in effect the heat energy our bodies generate can build up inside us, and so we feel uncomfortably hot. While changing our surface area can be reasonably effective, it isn’t as effective when the air temperature is too high or too low. However, the human body has plenty of other tricks up its sleeve.
Let’s look at another thing we do to maintain a constant body temperature and it has to do with our blood.

 Part C: Blood
Part C: Blood
You probably already know that the blood flowing in our blood vessels is a hugely important part of our bodies. Blood carries oxygen and glucose to all our cells and carries carbon dioxide and other wastes away from our cells. It also transports hormones and other important chemicals around our bodies.
Blood also helps us to maintain a constant body temperature. Blood can carry heat energy from one part of the body to the other. It’s kind of similar to a convection current in a beaker of water.

 Arteries carry blood away from the heart and veins carry blood back to the heart. Now our blood vessels have the ability to constrict and to dilate.
Arteries carry blood away from the heart and veins carry blood back to the heart. Now our blood vessels have the ability to constrict and to dilate.
A normal artery, for example, might look like this. When it constricts, it reduces its diameter and less blood flows through it. When it dilates, its internal diameter widens, and so more blood passes through it. Arteries actually have tiny muscles in them that allows them to do this. The processes are called vasoconstriction and vasodilation; vaso refers to vessel, as in blood vessel.
 So what’s all this got to do with maintaining a constant body temperature?
So what’s all this got to do with maintaining a constant body temperature?
Well, depending on how hot or cold it is and how or cold we feel, the arteries that carry blood to our skin and within our skin can constrict or dilate to change where the blood flows and this affects how much heat energy we lose and how much we retain.
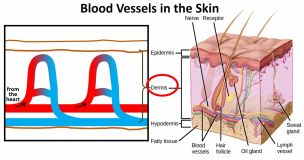 The skin is pretty complicated and contains hair follicles, sweat glands and other components, but in the very simple diagram on the left, I’m showing only the blood vessels. Initially, the blood flows from the heart to the skin along larger arteries and then flows into the main layer of our skin (called the dermis) via a series of loops. It
The skin is pretty complicated and contains hair follicles, sweat glands and other components, but in the very simple diagram on the left, I’m showing only the blood vessels. Initially, the blood flows from the heart to the skin along larger arteries and then flows into the main layer of our skin (called the dermis) via a series of loops. It  then returns to the heart through the veins. At normal temperatures when we’re not too hot and not too cold, but just right, the blood vessels (technically these smaller blood vessels are called capillaries) are just at their normal size. When we’re feeling hot however, the blood vessels that carry blood into our skin and the blood vessel loops that carry the blood closest to the outer surface of our skin dilate and the loops a little deeper down constrict. More blood therefore travels into the skin and more blood travels closer to the surface of the skin. This blood that is closer to the surface of the skin loses heat to the cool air around it and so it cools down a little. Remember, heat energy always travels from a hotter substance to a colder substance. The slightly cooler blood then goes back into our core to cool it down. What a brilliant system!
then returns to the heart through the veins. At normal temperatures when we’re not too hot and not too cold, but just right, the blood vessels (technically these smaller blood vessels are called capillaries) are just at their normal size. When we’re feeling hot however, the blood vessels that carry blood into our skin and the blood vessel loops that carry the blood closest to the outer surface of our skin dilate and the loops a little deeper down constrict. More blood therefore travels into the skin and more blood travels closer to the surface of the skin. This blood that is closer to the surface of the skin loses heat to the cool air around it and so it cools down a little. Remember, heat energy always travels from a hotter substance to a colder substance. The slightly cooler blood then goes back into our core to cool it down. What a brilliant system!
 The fact that more blood travels to the outer layers of our skin when we’re feeling hot results in the skin becoming redder.
The fact that more blood travels to the outer layers of our skin when we’re feeling hot results in the skin becoming redder.
It’s usually more noticeable in people that have lighter skin, but darker skin works in exactly the same way of course. The only real difference between human skin colours is that darker skin has more of a chemical called melanin in it!
 So what happens when we get cold? Well, when it’s cold, the arteries that carry blood to our skin constrict. This means that less blood flows to our skin and more blood stays in our core.
So what happens when we get cold? Well, when it’s cold, the arteries that carry blood to our skin constrict. This means that less blood flows to our skin and more blood stays in our core.
In our skin, the blood vessels nearer the surface constrict a lot. So less blood overall flows to the skin and a lot less goes near the skin surface. Since the skin is a good insulator, we don’t lose as much heat energy to the cold air around us.
 When people who have fair skin are feeling cold, they often go quite pale. This is because there’s less blood travelling to our skin. Once again, it’s not as obvious in darker skin.
When people who have fair skin are feeling cold, they often go quite pale. This is because there’s less blood travelling to our skin. Once again, it’s not as obvious in darker skin.
Our blood vessels are constantly adjusting as we move from place to place and as conditions change. Vasoconstriction and vasodilation play a major role in maintaining a constant body temperature. A part of the brain called the hypothalamus controls it all.
We sometimes go numb when we’re cold for the same reason. The body reduces the blood flow to our skin, and so the cells don’t get enough oxygen to function properly. This can sometimes make it difficult to perform simple tasks like installing a battery into a device.

 Elephants also use their blood to carry heat away from the insides of their bodies to the air outside. Elephant skin is very thick, so when they’re feeling hot, the arteries in their ears dilate and more blood is pumped through their ears which, unlike the rest of their body, have thin skin. Their ears also have a very large surface area. The blood cools down fairly quickly and it then returns into the body to cool the rest of the body. In fact all mammals use blood to help regulate their temperature.
Elephants also use their blood to carry heat away from the insides of their bodies to the air outside. Elephant skin is very thick, so when they’re feeling hot, the arteries in their ears dilate and more blood is pumped through their ears which, unlike the rest of their body, have thin skin. Their ears also have a very large surface area. The blood cools down fairly quickly and it then returns into the body to cool the rest of the body. In fact all mammals use blood to help regulate their temperature.
Now when it’s really hot, like it is now, or really cold, adjusting the way the blood flows in our bodies isn’t effective enough at maintaining a constant body temperature for very long. But our bodies cope in more extreme conditions by sweating when it’s really hot and shivering when it’s really cold. How do these actions help? Let’s take a look.
Part D: Sweating and Shivering
 If our internal body temperature drops too much when we’re caught out in the cold, and I’m talking of a drop in core body temperature of less than 1°C, then our bodies generate more heat by shivering.
If our internal body temperature drops too much when we’re caught out in the cold, and I’m talking of a drop in core body temperature of less than 1°C, then our bodies generate more heat by shivering.
If we’re actually doing exercise on a cold day—it was 6°C on this particular day—then our muscles usually produce enough heat to stay warm without shivering of course. We can also rub our hands together to generate heat. However, if we’re standing still and our body temperature starts dropping, we start to shiver; our muscles contract and then relax, contract and then relax over and over really really quickly. Shivering generates more heat which keeps our bodies at the right temperature. Our teeth often start to chatter because the muscles that control our jaws are also shivering. Anyway, I need to get to a heater or to time travel into summer.
That’s better. If it’s really hot and our bodies start to overheat, then we start to sweat.

 Our skin is loaded with millions of tiny sweat glands and when we’re hot, the sweat glands squeeze sweat (which is more than 99% water) out onto the skin surface.
Our skin is loaded with millions of tiny sweat glands and when we’re hot, the sweat glands squeeze sweat (which is more than 99% water) out onto the skin surface.
But the sweat starts off at the same temperature as the skin. How does it cool us down? The act of sweating itself doesn’t cool the body but the body cools down as the sweat evaporates.

 The air in this room is about 25°C and the water in this glass is also 25°C.
The air in this room is about 25°C and the water in this glass is also 25°C.
If I wet a small piece of a tissue and place it over the thermometer, the temperature of the water starts to drop. Within about a minute, the temperature was down to 22°C, and about 2 minutes after that it got down to 19°C. The rapid evaporation of the water from the thin layer of tissue cools the water and therefore the thermometer that the water is touching also cools down.
If I place the thermometer in the breeze created by a fan, the temperature drops even more (from 19°C to about 16°C). This is because the breeze causes the water to evaporate even faster, and so the cooling effect is greater. So the water started at 25°C but got down to 16°C as it evaporated.
 But how does evaporation create a cooling effect? Well it’s time to bring the Kinetic Theory of Matter back into play. I wonder how many times I’ve mentioned the Kinetic Theory so far in this series!
But how does evaporation create a cooling effect? Well it’s time to bring the Kinetic Theory of Matter back into play. I wonder how many times I’ve mentioned the Kinetic Theory so far in this series!
The Kinetic Theory of Matter (did it again) says that in water, the water molecules are bouncing around and bumping into each other continuously.
In cooler water, they move, on average, more slowly than they do in warmer water. But the key thing here is that all the water molecules regardless of the temperature are moving at different speeds.
Quite often, just through random collisions, water molecules that are already moving faster than the average will be bumped into and sent flying off into the air. That’s evaporation. But if it’s the fastest molecules that are more likely to evaporate, what happens to the average speed of the molecules that are left? Well, the average speed drops, since the faster ones are evaporating. Now if the average speed drops because the faster molecules are evaporating, then the water becomes cooler. It’s simple logic!
This now cooler water is cooler than whatever it’s touching and so it cools whatever it’s touching down.
 Now as sweat evaporates, it cools the skin down. Heat energy conducts from the skin into the sweat and then the energy is taken away by the evaporating water molecules. This happens continuously. Of course the blood that is near the surface of the skin also cools down—much more than if there wasn’t any sweat—and it then moves into the rest of the body to cool it down as well.
Now as sweat evaporates, it cools the skin down. Heat energy conducts from the skin into the sweat and then the energy is taken away by the evaporating water molecules. This happens continuously. Of course the blood that is near the surface of the skin also cools down—much more than if there wasn’t any sweat—and it then moves into the rest of the body to cool it down as well.
So as I said, it’s not the sweating itself that cools us down, it’s the evaporation of the sweat that cools us down.

 We can actually lose quite a lot of water through sweating when it’s hot. It’s 32°C right now and I’m going to do some exercise to show you how much. My weight before exercising was 85.1 kg. I did about two hours of running, cycling, and weight training during which I sweated a lot and I didn’t drink any water. After the exercise, my weight went down to 82.9 kg, a loss of about 2.2 kg, most of which was the water that was in my sweat. However, a small part of the loss was from burning off fat and glucose (that is, the fuel I was using) which I then breathed out through my mouth. Just like a car burns petrol in the engine and the waste products come out of the exhaust pipe, humans burn fat and glucose and we breathe out all of the waste carbon dioxide. Our lungs, trachea, mouth, and nose make up our exhaust pipes. But let’s get back to sweat.
We can actually lose quite a lot of water through sweating when it’s hot. It’s 32°C right now and I’m going to do some exercise to show you how much. My weight before exercising was 85.1 kg. I did about two hours of running, cycling, and weight training during which I sweated a lot and I didn’t drink any water. After the exercise, my weight went down to 82.9 kg, a loss of about 2.2 kg, most of which was the water that was in my sweat. However, a small part of the loss was from burning off fat and glucose (that is, the fuel I was using) which I then breathed out through my mouth. Just like a car burns petrol in the engine and the waste products come out of the exhaust pipe, humans burn fat and glucose and we breathe out all of the waste carbon dioxide. Our lungs, trachea, mouth, and nose make up our exhaust pipes. But let’s get back to sweat.
 According to the scales, this is about how much I’ve sweated over the past few hours. If you do any kind of sport on hot days, you have to drink plenty of water.
According to the scales, this is about how much I’ve sweated over the past few hours. If you do any kind of sport on hot days, you have to drink plenty of water.
Quite often, you don’t notice as much sweat when you’re exercising because it evaporates almost as soon as it comes out onto your skin. When you stop exercising, and the air isn’t sweeping past you any more, you seem to sweat more, but you aren’t really, it’s just that the sweat isn’t evaporating as quickly anymore.
When we exercise, we generate more heat than we normally do anyway and this extra heat has to be removed from our bodies. Our evaporating sweat is responsible for the removal of most of this extra heat energy.
 This simple experiment shows just how effective sweating is. This heater is blowing out air at a temperature of about 70°C but the thermometer wrapped in a wet tissue is staying at a relatively cool 28°C or so because of the evaporating water. Sweating, well the evaporation of sweat, is very effective even in extreme temperatures way hotter than human body temperature as long as you have enough water to drink of course.
This simple experiment shows just how effective sweating is. This heater is blowing out air at a temperature of about 70°C but the thermometer wrapped in a wet tissue is staying at a relatively cool 28°C or so because of the evaporating water. Sweating, well the evaporation of sweat, is very effective even in extreme temperatures way hotter than human body temperature as long as you have enough water to drink of course.
In really hot weather, a lot of people use fans to cool down, and they can be really effective even though they don’t actually cool the air in a room.
 Fans make us feel cooler not because they cool the air in the room but mostly because they make sweat evaporate faster. If sweat evaporates but the water molecules stay near our skin because there’s no air movement, it makes it hard for more sweat to evaporate. A fan blows away the warm, moist air that builds up around us when we’re sitting still so that more sweat can evaporate. If you plan to leave the room for any amount of time, there’s no point leaving the fan on because, as I said, the fan cools you down by helping the sweat to evaporate faster; fans don’t cool the air.
Fans make us feel cooler not because they cool the air in the room but mostly because they make sweat evaporate faster. If sweat evaporates but the water molecules stay near our skin because there’s no air movement, it makes it hard for more sweat to evaporate. A fan blows away the warm, moist air that builds up around us when we’re sitting still so that more sweat can evaporate. If you plan to leave the room for any amount of time, there’s no point leaving the fan on because, as I said, the fan cools you down by helping the sweat to evaporate faster; fans don’t cool the air.
Also, the sweat that has evaporated has to leave the room or else the build up of moisture in the air will just restrict how easily more sweat will evaporate, so it’s a good idea to leave a window or a door slightly open.
Air conditioners actually cool the air so they don’t rely on our sweat, and they remove the moisture from the air as well which is called dehumidifying the air.
When it’s windy, or when we are moving through the air, sweat evaporates faster, so it has a greater cooling effect.
 When it’s “humid”, there’s lots of moisture in the air already and so the sweat doesn’t evaporate as easily. There’s only so much moisture the air can hold. A high humidity reduces the rate of evaporation and so the cooling effect is reduced. People often say it’s “very muggy today” or “it’s very sticky”. The sweat builds up without evaporating a whole lot.
When it’s “humid”, there’s lots of moisture in the air already and so the sweat doesn’t evaporate as easily. There’s only so much moisture the air can hold. A high humidity reduces the rate of evaporation and so the cooling effect is reduced. People often say it’s “very muggy today” or “it’s very sticky”. The sweat builds up without evaporating a whole lot.
By the way, sweat is completely odourless, well, fresh sweat is anyway. The smell associated with sweat is caused by bacteria that feed on the tiny tiny amount of protein in the sweat. The bacteria produce certain chemicals that can sometimes be a little smelly.
 Dogs don’t have many sweat glands, and so they cool down by panting. As the saliva on their tongues and in their mouths evaporates (well, the water that the saliva is mostly made of evaporates) it cools the blood just under the surface and this cooler blood then moves back into the rest of their bodies.
Dogs don’t have many sweat glands, and so they cool down by panting. As the saliva on their tongues and in their mouths evaporates (well, the water that the saliva is mostly made of evaporates) it cools the blood just under the surface and this cooler blood then moves back into the rest of their bodies.
I actually find it amazing that all living things—we’ve looked at dogs, humans, and elephants, but there are millions of other examples—can make use of the physics of heat and motion, without even being aware of it, to survive in whatever habitat they live in. The biological world that we are a part of can only exist in a physical world that allows it to.
So if water didn’t evaporate or if it didn’t cool down as it evaporated, then humans just wouldn’t sweat, because there would be no point having the ability to sweat if there was no benefit to it! We would need to rely on different mechanisms to cool us down when we were hot. Our biology is governed by the Laws of Physics. To take another example, if the thermal conductivities of skin and feathers and fat were different then all animals would be different as well.
The biological world, that is, all life on Earth, is shaped by the Physics (and Chemistry) of the physical world.
Now our ability to maintain a constant core temperature is limited. If we’re exposed to hot or cold conditions for too long we can get hyperthermia (our body temperature rises too much) or hypothermia (our body temperature drops too much) and both can spell big trouble. Lethal trouble in fact.
 Part E: Hypothermia and Hyperthermia
Part E: Hypothermia and Hyperthermia
If you’re out in the cold for too long without appropriate clothing, your body temperature will eventually drop to below its normal temperature range (37°C ± 0.5°C) and you get what is called hypothermia. Hypo (???) means under in Greek, so the word hypothermia means underheat. (A hypodermic needle is a needle that is inserted under your skin; hypo is under, derma is Greek for skin, hence hypodermic.)
 If your core temperature reaches 36°C you’ll be shivering uncontrollably and if it drops below about 35°C your speech becomes blurred, you become unco-ordinated, and you start to lose your reasoning ability. All these temperature figures are approximate because everyone’s different. As your core temperature drops, your temperature-regulation systems stop working and in fact you reach a point where you stop shivering. Many people suffering severe hypothermia start complaining that it’s too hot! In fact many people who have died of hypothermia had taken their clothes off before they died, no doubt thinking that this was going to help them. Below a core temperature of about 28°C, the heart stops beating resulting of course in death.
If your core temperature reaches 36°C you’ll be shivering uncontrollably and if it drops below about 35°C your speech becomes blurred, you become unco-ordinated, and you start to lose your reasoning ability. All these temperature figures are approximate because everyone’s different. As your core temperature drops, your temperature-regulation systems stop working and in fact you reach a point where you stop shivering. Many people suffering severe hypothermia start complaining that it’s too hot! In fact many people who have died of hypothermia had taken their clothes off before they died, no doubt thinking that this was going to help them. Below a core temperature of about 28°C, the heart stops beating resulting of course in death.
The time it takes to get hypothermia depends of course on the conditions, what you’re wearing, and factors such as how big you are and your fitness level.
In cold windy conditions it can take less than an hour if you’re not appropriately dressed.

In the snow, where the air temperature was about 0°C, it took me only about fifteen minutes of standing around in jeans and a T-shirt to get to the point where I shivering uncontrollably. My core temperature actually dropped down to below 36°C. Another fifteen minutes and I could have been in real danger. After shooting this scene I went back to the car which was only about 100 metres away, turned on the heater and drank hot tea to warm up.
In 15°C still air, it can take 3-4 hours to get hypothermia if for some reason you’re standing around in shorts and a T-shirt. Obviously the colder it is and the windier it is, the less time it takes.
 In 15°C water though, if you’re just wearing bathers, you’ll have severe hypothermia within about an hour, and there’s a really high chance of dying within a few hours. This is because water conducts heat away from your body about 25 times faster than air does.
In 15°C water though, if you’re just wearing bathers, you’ll have severe hypothermia within about an hour, and there’s a really high chance of dying within a few hours. This is because water conducts heat away from your body about 25 times faster than air does.
The arteries delivering blood to your arms and legs constrict so that your body can minimize the heat loss from these body parts and keep more heat within your inner core, which includes the heart and the brain. As a result, the muscles in your arms and legs get very very weak which makes swimming very difficult.
 Being exposed to water at temperatures of 15°C is far more dangerous than being exposed to air at a temperature of 15°C.
Being exposed to water at temperatures of 15°C is far more dangerous than being exposed to air at a temperature of 15°C.
In 0°C water, most people will die within about 30 minutes, but not necessarily from hypothermia. In many cases, people who fall into ice-cold water, like many of the passengers did when the Titanic famously sank after striking an iceberg in the North Atlantic ocean in 1912, die by drowning rather than from hypothermia even if they’re good swimmers.
Many people, on sudden exposure to ice-cold water, undergo what’s called cold shock. Now this isn’t ice-cold water, and I’m obviously wearing a wet suit but this is kind of what happens.
Now before I continue, I’m about to act out dying again. I’m not claiming to be a good actor or anything but people have actually died in similar circumstances to what you’re about to see, so, for some of you watching, it might be confronting. Anyway, let’s see what sometimes happens when people undergo cold shock.


 They start to hyperventilate uncontrollably and if their head goes under they breathe in water and this causes them to drown almost immediately, I’m talking seconds. In this scenario, it would be just as likely that having gone under when I first hit the water, I would never come up again, because I would hyperventilate while underwater before I even had a chance to resurface.
They start to hyperventilate uncontrollably and if their head goes under they breathe in water and this causes them to drown almost immediately, I’m talking seconds. In this scenario, it would be just as likely that having gone under when I first hit the water, I would never come up again, because I would hyperventilate while underwater before I even had a chance to resurface.

Many also die from a heart attack.
How’s my acting? The blood that is in their skin when they hit the water cools down really quickly and then on returning to the heart causes the heart to stop beating because the heart muscle doesn’t work properly if it’s not warm.
If you manage to avoid the initial cold shock and the heart attack, which many people don’t, you’re likely to drown within 15-30 minutes even if you’re a good swimmer.
This is because the body, in a desperate attempt to maintain heat in the core, almost completely cuts off the blood supply to the muscles in your arms and legs making you unable to actually swim. If you’re wearing a life jacket that allows you to keep your head above water, then you’ll survive a little longer, but not really much longer.
 The only way to save a person suffering from severe hypothermia, before they die of course, is to get them out of the cold and warm them up, slowly, with an external heat source, like a heater and/or by giving them a hot drink. An external heat source, rather than just a blanket, say, is usually necessary in severe cases because the person may have actually lost the ability to regulate their temperature. Going to a hospital may be necessary.
The only way to save a person suffering from severe hypothermia, before they die of course, is to get them out of the cold and warm them up, slowly, with an external heat source, like a heater and/or by giving them a hot drink. An external heat source, rather than just a blanket, say, is usually necessary in severe cases because the person may have actually lost the ability to regulate their temperature. Going to a hospital may be necessary.
If you’re out in the heat too long, your core body temperature may rise above 37°C and you get hyperthermia. Hyper comes from the Greek word for “above”. The word hyperventilate also uses the prefix “hyper”.
 Hyperthermia is also called heatstroke. Your core temperature only needs to get to about 38°C before you start getting headaches, nausea, and dizziness and it gets worse the hotter your core gets. If you’re in a hot environment and your core gets to 40°C, movement becomes difficult and you are extremely close to death. Very few survive if their core reaches 42°C.
Hyperthermia is also called heatstroke. Your core temperature only needs to get to about 38°C before you start getting headaches, nausea, and dizziness and it gets worse the hotter your core gets. If you’re in a hot environment and your core gets to 40°C, movement becomes difficult and you are extremely close to death. Very few survive if their core reaches 42°C.
Now it’s probably pretty obvious, but to treat someone suffering from hyperthermia, you have to just get them out of the heat and find them a cool place to sit and rest. They’ll need to drink water and in severe cases an ambulance may have to be called.
 So, in summary, maintaining a constant core temperature requires our bodies to balance how much heat energy we’re producing within our bodies combined with how much we’re receiving from an external source (from, for example, the sun’s rays) with how much heat energy we’re losing to, for example, cool air that’s around us. The balance has to be just right!
So, in summary, maintaining a constant core temperature requires our bodies to balance how much heat energy we’re producing within our bodies combined with how much we’re receiving from an external source (from, for example, the sun’s rays) with how much heat energy we’re losing to, for example, cool air that’s around us. The balance has to be just right!
If we’re too cold (which means that more heat is being lost than what is being gained) then our bodies can generate more heat by exercising or shivering or they slow the rate of heat loss by reducing our surface area and keeping blood away from the outer surface of the skin.
If we’re too hot (which means we’re either generating too much heat or receiving too much heat and we’re not losing enough heat), we can generate less heat by resting and our bodies can shed any excess heat by sweating (which of course requires lots of water), by increasing our surface area and by pumping more blood to the outer surface of our skin.
Obviously the technology we have like heaters, clothing, and air conditioners help enormously and we can cool down for example by going to the beach or whatever, but in this video I’ve concentrated mostly on what our bodies themselves do to regulate our temperature.
Of course, a lot of what we do is governed by what season we’re in. Seasonal changes in temperature affect everything on the planet including, for example, what we wear and plant and animal life cycles. But what causes the seasons? Why do we have summer, autumn, winter, and spring? Well, that’s what we’ll be looking at in our next episode. See you then.
CREDITS:
![]() Water molecule simulations were created by PhET Interactive Simulations, University of Colorado, Boulder, http://phet.colorado.edu. Licensed under CC-BY.
Water molecule simulations were created by PhET Interactive Simulations, University of Colorado, Boulder, http://phet.colorado.edu. Licensed under CC-BY.
![]() https://psychonautwiki.org/wiki/File:Vasodilation_and_vasoconstriction.png by Josikins Creative Commons Attribution-ShareAlike 4.0 International
https://psychonautwiki.org/wiki/File:Vasodilation_and_vasoconstriction.png by Josikins Creative Commons Attribution-ShareAlike 4.0 International
![]() File:Blausen 0803 Skin FreeNerveEndings.png by BruceBlaus is licensed under the Creative Commons Attribution 3.0 Unported license.
File:Blausen 0803 Skin FreeNerveEndings.png by BruceBlaus is licensed under the Creative Commons Attribution 3.0 Unported license.
![]() Aug 02, 2017- Sunrise- Jamie demonstrates the Thermal Camera by Safari Chey. Creative Commons Attribution license
Aug 02, 2017- Sunrise- Jamie demonstrates the Thermal Camera by Safari Chey. Creative Commons Attribution license
![]() Ambulance on King William St (https://www.youtube.com/watch?v=Fdnnjf2A58k) by RS 1990. Creative Commons Attribution license
Ambulance on King William St (https://www.youtube.com/watch?v=Fdnnjf2A58k) by RS 1990. Creative Commons Attribution license
![]() Blood Flow in the Human Body (https://www.youtube.com/watch?v=GwX41xm9esY) by
Blood Flow in the Human Body (https://www.youtube.com/watch?v=GwX41xm9esY) by
Forsyth Tech CTL. Creative Commons Attribution license



